Home » QA & Regulatory

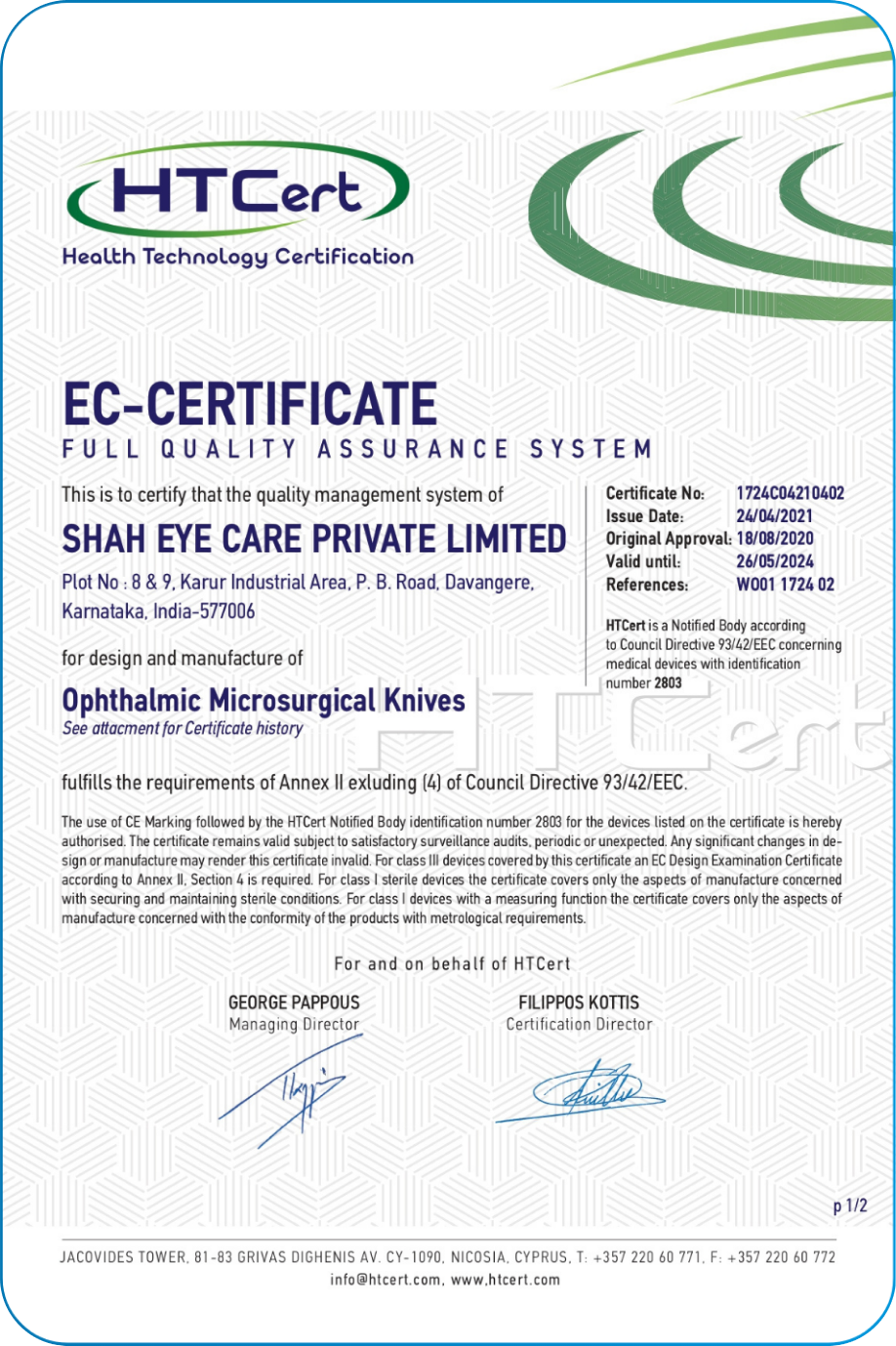
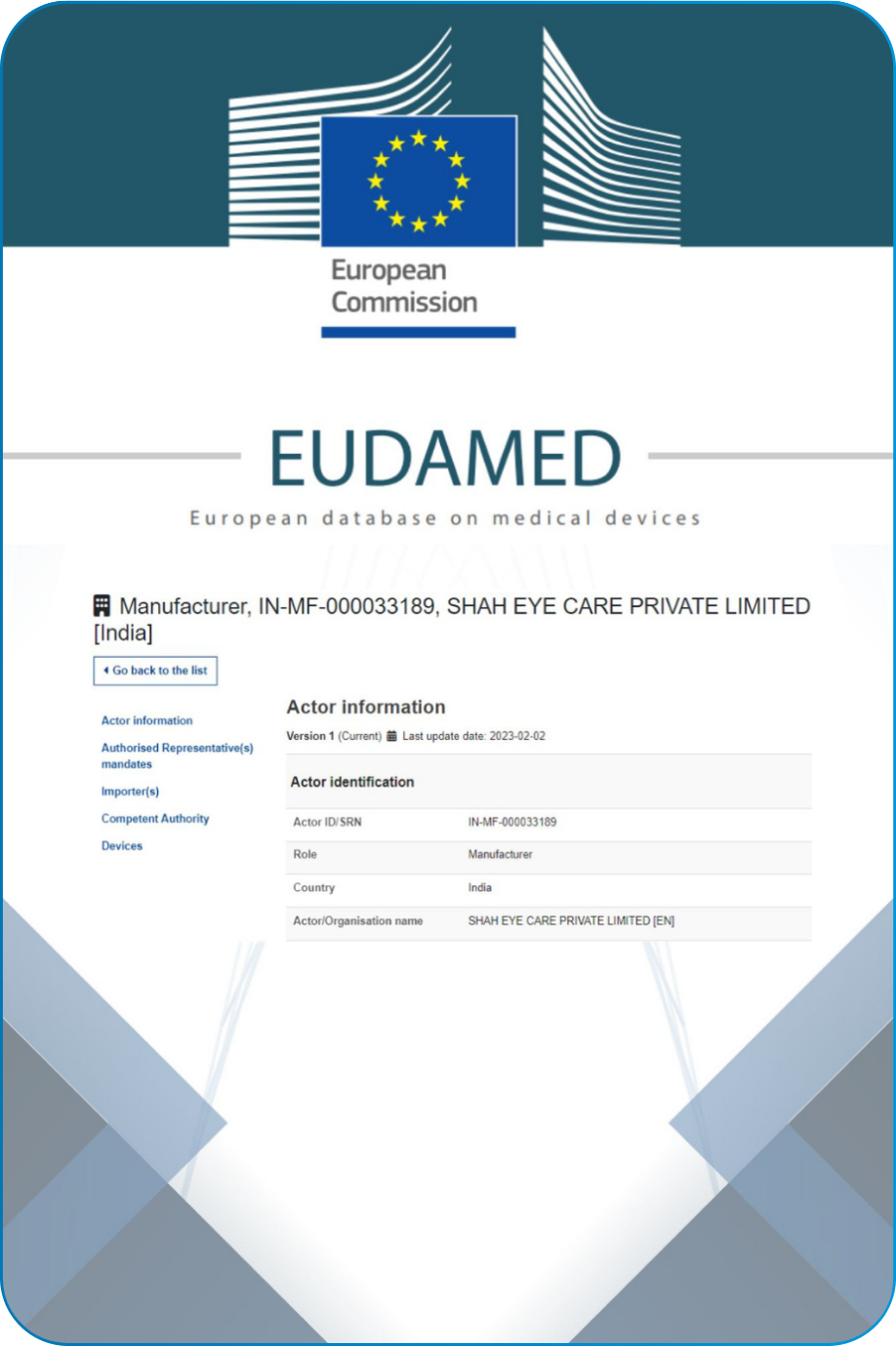
Our Quality system follows the ISO 13485, 21 CFR part 820 and Fifth Schedule of Medical Device Rule-2017.Navigating the complex landscape of regulatory requirements is fundamental commitment to compliance. This encompasses global regulations USFDA, CE, CDSCO and EUDAMED.